Contact Us
By clicking Submit, you confirm that you accept our Privacy Policy and that you agree to your personal contact information being used to contact you and added to our database. If at any time you wish your personal information to be removed from the American Regent® database, please submit a message request to corpcommunications@americanregent.com.
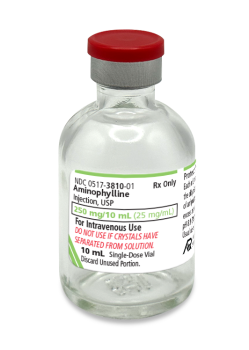
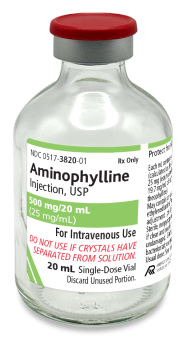
Aminophylline Injection, USP
25 mg/mL Aminophylline, Dihydrate
(Equivalent to 19.7 mg/mL of Anhydrous Theophylline)
For intravenous administration
INDICATIONS AND USAGE
Intravenous theophylline is indicated as an adjunct to inhaled beta-2 selective agonists and systemically administered corticosteroids for the treatment of acute exacerbations of the symptoms and reversible airflow obstruction associated with asthma and other chronic lung diseases, e.g., emphysema and chronic bronchitis.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Aminophylline is contraindicated in patients with a history of hypersensitivity to theophylline or other components in the product including ethylenediamine.
WARNINGS
Concurrent Illness: Theophylline should be used with extreme caution in patients with the following clinical conditions due to the increased risk of exacerbation of the concurrent condition: Active peptic ulcer disease; Seizure disorders; Cardiac arrhythmias (not including bradyarrhythmias)
Conditions That Reduce Theophylline Clearance:
There are several readily identifiable causes of reduced theophylline clearance. If the infusion rate is not appropriately reduced in the presence of these risk factors, severe and potentially fatal theophylline toxicity can occur. Careful consideration must be given to the benefits and risks of theophylline use and the need for more intensive monitoring of serum theophylline concentrations in patients with the following risk factors:
Age: Neonates (term and premature) Children <1 year; Elderly (>60 years)
Concurrent Diseases: Acute pulmonary edema; Congestive heart failure; Cor pulmonale; Fever; ≥102° for 24 hours or more, or lesser temperature elevations for longer periods; Hypothyroidism; Liver disease; cirrhosis, acute hepatitis; Reduced renal function in infants <3 months of age; Sepsis with multi-organ failure; Shock
Cessation of Smoking Drug Interactions: Adding a drug that inhibits theophylline metabolism or stopping a concurrently administered drug that enhances theophylline metabolism
When Signs or Symptoms of Theophylline Toxicity Are Present: Whenever a patient receiving theophylline develops nausea or vomiting, particularly repetitive vomiting, or other signs or symptoms consistent with theophylline toxicity (even if another cause may be suspected), the intravenous infusion should be stopped and a serum theophylline concentration measured immediately.
Dosage Increases: Increases in the dose of intravenous theophylline should not be made in response to an acute exacerbation of symptoms unless the steady-state serum theophylline concentration is <10 mcg/mL.
PRECAUTIONS
General: Careful consideration of the various interacting drugs and physiologic conditions that can alter theophylline clearance and require dosage adjustment should occur prior to initiation of theophylline therapy and prior to increases in theophylline dose.
Monitoring Serum Theophylline Concentrations: Serum theophylline concentration measurements should be used to determine whether the dosage is appropriate.
Effects on Laboratory Tests: As a result of its pharmacological effects, theophylline at serum concentrations within the 10 - 20 mcg/mL range modestly increases plasma glucose (from a mean of 88 mg% to 98 mg%), uric acid (from a mean of 4 mg/dl to 6 mg/dl), free fatty acids (from a mean of 451 μEq/L to 800 μEq/L), total cholesterol (from a mean of 140 vs 160 mg/dl), HDL (from a mean of 36 to 50 mg/dl), HDL/LDL ratio (from a mean of 0.5 to 0.7), and urinary free cortisol excretion (from a mean of 44 to 63 mcg/24 hr). Theophylline at serum concentrations within the 10 - 20 mcg/mL range may also transiently decrease serum concentrations of triiodothyronine (144 before, 131 after one week and 142 ng/dl after 4 weeks of theophylline). The clinical importance of these changes should be weighed against the potential therapeutic benefit of theophylline in individual patients.
Drug Interactions: Theophylline interacts with a wide variety of drugs. The interaction may be pharmacodynamic or pharmacokinetic.
USE IN SPECIFIC POPULATIONS
Pregnancy: There are no adequate and well controlled studies in pregnant women.
Nursing Mothers: Theophylline is excreted into breast milk and may cause irritability or other signs of mild toxicity in nursing human infants. The concentration of theophylline in breast milk is about equivalent to the maternal serum concentration.
Pediatric Use: Theophylline is safe and effective for the approved indications in pediatric patients. The constant infusion rate of intravenous theophylline must be selected with caution in pediatric patients since the rate of theophylline clearance is highly variable across the age range of neonates to adolescents. Due to the immaturity of theophylline metabolic pathways in pediatric patients under the age of one year, particular attention to dosage selection and frequent monitoring of serum theophylline concentrations are required when theophylline is prescribed to pediatric patients in this age group.
Geriatric Use: Elderly patients are at significantly greater risk of experiencing serious toxicity from theophylline than younger patients due to pharmacokinetic and pharmacodynamic changes associated with aging. Theophylline clearance is reduced in patients greater than 60 years of age, resulting in increased serum theophylline concentrations in response to a given theophylline infusion rate.
ADVERSE REACTIONS
Adverse reactions associated with theophylline are generally mild when peak serum theophylline concentrations are <20 mcg/mL and mainly consist of transient caffeine-like adverse effects such as nausea, vomiting, headache, and insomnia. When peak serum theophylline concentrations exceed 20 mcg/mL, theophylline produces a wide range of adverse reactions including persistent vomiting, cardiac arrhythmias, and intractable seizures which can be lethal.
Other adverse reactions that have been reported at serum theophylline concentrations <20 mcg/mL include diarrhea, irritability, restlessness, fine skeletal muscle tremors, and transient diuresis. In patients with hypoxia secondary to COPD, multifocal atrial tachycardia and flutter have been reported at serum theophylline concentrations ≥15 mcg/mL. There have been a few isolated reports of seizures at serum theophylline concentrations <20 mcg/mL in patients with an underlying neurological disease or in elderly patients. The occurrence of seizures in elderly patients with serum theophylline concentrations <20 mcg/mL may be secondary to decreased protein binding resulting in a larger proportion of the total serum theophylline concentration in the pharmacologically active unbound form. The clinical characteristics of the seizures reported in patients with serum theophylline concentrations <20 mcg/mL have generally been milder than seizures associated with excessive serum theophylline concentrations resulting from an overdose (i.e., they have generally been transient, often stopped without anticonvulsant therapy, and did not result in neurological residua).
Products containing aminophylline may rarely produce severe allergic reactions of the skin, including exfoliative dermatitis, after systemic administration in a patient who has been previously sensitized by topical application of a substance containing ethylenediamine. In such patient’s skin patch tests are positive for ethylenediamine, a component of aminophylline, and negative for theophylline. Pharmacists and other individuals who experience repeated skin exposure while physically handling aminophylline may develop a contact dermatitis due to the ethylenediamine component.
OVERDOSAGE
General: The chronicity and pattern of theophylline overdosage significantly influences clinical manifestations of toxicity, management and outcome. There are two common presentations: 1) acute overdose, i.e., infusion of an excessive loading dose or excessive maintenance infusion rate for less than 24 hours, and 2) chronic overdosage, i.e., excessive maintenance infusion rate for greater than 24 hours.
Several studies have described the clinical manifestations of theophylline overdose following oral administration and attempted to determine the factors that predict life-threatening toxicity. In general, patients who experience an acute overdose are less likely to experience seizures than patients who have experienced a chronic overdosage, unless the peak serum theophylline concentration is >100 mcg/mL. After a chronic overdosage, generalized seizures, life-threatening cardiac arrhythmias, and death may occur at serum theophylline concentrations >30 mcg/mL. The severity of toxicity after chronic overdosage is more strongly correlated with the patient's age than the peak serum theophylline concentration; patients >60 years are at the greatest risk for severe toxicity and mortality after a chronic overdosage. Pre-existing or concurrent disease may also significantly increase the susceptibility of a patient to a particular toxic manifestation, e.g., patients with neurologic disorders have an increased risk of seizures and patients with cardiac disease have an increased risk of cardiac arrhythmias for a given serum theophylline concentration compared to patients without the underlying disease.
Other manifestations of theophylline toxicity include increases in serum calcium, creatine kinase, myoglobin and leukocyte count, decreases in serum phosphate and magnesium, acute myocardial infarction, and urinary retention in men with obstructive uropathy.
Seizures associated with serum theophylline concentrations >30 mcg/mL are often resistant to anticonvulsant therapy and may result in irreversible brain injury if not rapidly controlled. Death from theophylline toxicity is most often secondary to cardiorespiratory arrest and/or hypoxic encephalopathy following prolonged generalized seizures or intractable cardiac arrhythmias causing hemodynamic compromise.
Refer to the full prescribing information for information on overdose management.
For additional Safety Information, please see Full Prescribing Information.
You are encouraged to report adverse drug events to American Regent, Inc. at 1-800-734-9236 or to the FDA
by visiting www.fda.gov/medwatch or calling 1-800-FDA-1088.
REF-2453 3/2023