Atropine SulfateInjection, USP
- Preservative-free
- Vial closure is not made with natural rubber latex
- This drug product is not FDA-approved
- These marketed unapproved Atropine products have been replaced with NDC 0157-1004-25 and 0517-1001-25. All future orders will be filled with FDA-approved Atropine NDC 0517-1004-25 and 0517-1001-25. Marketed unapproved Atropine (NDC 0517-0401-25 and 05171010-25) can remain in your inventory. There is no need to return the product, and they are suitable for use through expiry unless a situation arises to warrant a return.
For more information on FDA-approved Atropine Sulfate Injection, USP click here.
| Pack NDC# | Strength | Supplied as | Shelf pack | Vial opening size | Product info | Availability | ||
|---|---|---|---|---|---|---|---|---|
|
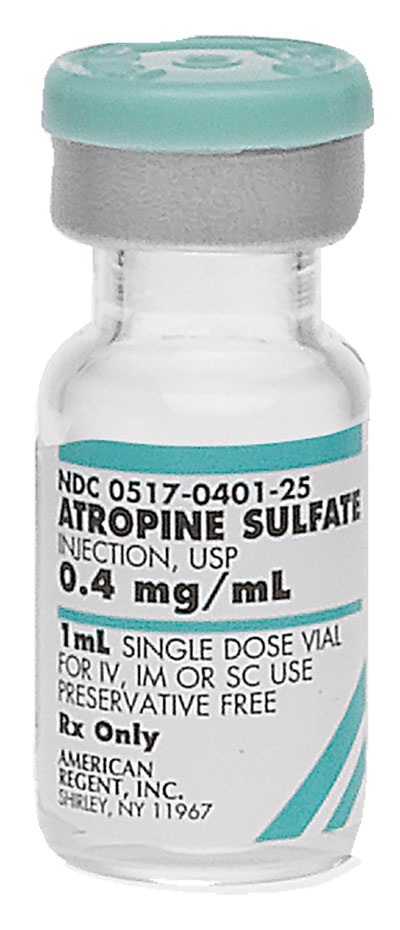
Strength:
0.4 mg/mL Supplied As:1 mL |
0517-0401-25 |
0.4 mg/mL |
1 mL |
25 | 13 mm | Product Label Full Prescribing Information Safety Data Sheet | Discontinued | |
Product Label Full Prescribing Information Safety Data Sheet Wholesaler Numbers
Case Information
|
||||||||
|
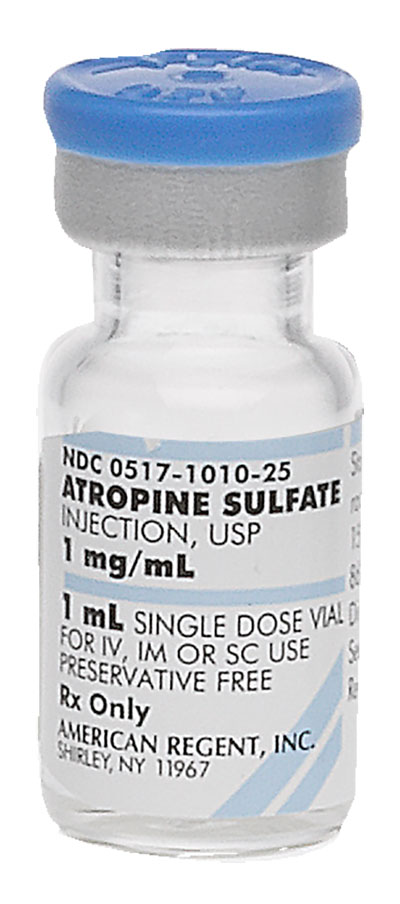
Strength:
1 mg/mL Supplied As:1 mL |
0517-1010-25 |
1 mg/mL |
1 mL |
25 | 13 mm | Product Label Full Prescribing Information Safety Data Sheet | Discontinued | |
Product Label Full Prescribing Information Safety Data Sheet Wholesaler Numbers
Case Information
|
||||||||
Contact Us
-
P: 800-645-1706
(8:00am–6pm Monday - Thursday and Friday 8:00am–4pm Eastern Time) -
P: 800-734-9236
F: 610-650-0170Adverse Drug Events (ADEs) may be reported to the FDA:
Phone: 1-800-FDA-1088
Web: www.fda.gov/medwatch -
P: 888-354-4859
-
P: 888-354-4855
(9am–5pm Eastern Time, Monday - Friday) -
American Regent, Inc.
5 Ramsey Road
Shirley, NY 11967P: 631-924-4000
F: 631-924-1731
By clicking Submit, you confirm that you accept our Privacy Policy and that you agree to your personal contact information being used to contact you and added to our database. If at any time you wish your personal information to be removed from the American Regent® database, please submit a message request to corpcommunications@americanregent.com.