Contact Us
By clicking Submit, you confirm that you accept our Privacy Policy and that you agree to your personal contact information being used to contact you and added to our database. If at any time you wish your personal information to be removed from the American Regent® database, please submit a message request to corpcommunications@americanregent.com.
CONTRAINDICATIONS
Vasopressin injection is contraindicated in patients with known allergy or hypersensitivity to 8-L-arginine vasopressin or chlorobutanol.
WARNINGS AND PRECAUTIONS
Worsening Cardiac Function: – A decrease in cardiac index may be observed with the use of vasopressin.
Reversible Diabetes Insipidus: - Patients may experience reversible diabetes insipidus, manifested by the development of polyuria, a dilute urine, and hypernatremia after cessation of treatment with vasopressin.
ADVERSE REACTIONS
The following adverse reactions associated with the use of vasopressin were identified in the literature. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to estimate their frequency reliably or to establish a causal relationship to drug exposure:
Bleeding/lymphatic system disorders: Hemorrhagic shock, decreased platelets, intractable bleeding
Cardiac disorders: Right heart failure, atrial fibrillation, bradycardia, myocardial ischemia
Gastrointestinal disorders: Mesenteric ischemia
Hepatobiliary: Increased bilirubin levels
Renal/urinary disorders: Acute renal insufficiency
Vascular disorders: Distal limb ischemia
Metabolic: Hyponatremia
Skin: Ischemic lesions
DRUG INTERACTIONS
Pressor effects of catecholamines and vasopressin injection are expected to be additive.
Indomethacin may prolong the effects of vasopressin injection.
Use with ganglionic blocking agents may increase the effect of vasopressin injection on mean arterial blood pressure.
Use with drugs suspected of causing SIADH (Syndrome of Inappropriate Antidiuretic Hormone Secretion) may increase the pressor effect in addition to the antidiuretic effect of vasopressin injection.
Co-administration of drugs suspected of causing diabetes insipidus may decrease the pressor response in addition to the antidiuretic effect of vasopressin injection.
USE IN SPECIFIC POPULATIONS
Pregnancy: May induce tonic uterine contractions.
Pediatric Use: Safety and effectiveness have not been established.
Geriatric Use: Dose selection for an elderly patient should be cautious.
OVERDOSAGE
Overdosage with vasopressin injection can be expected to manifest as consequences of vasoconstriction of various vascular beds (peripheral, mesenteric, and coronary) and as hyponatremia. In addition, overdosage may lead less commonly to ventricular tachyarrhythmias (including Torsade de Pointes), rhabdomyolysis, and non-specific gastrointestinal symptoms.
Direct effects will resolve within minutes of withdrawal of treatment.
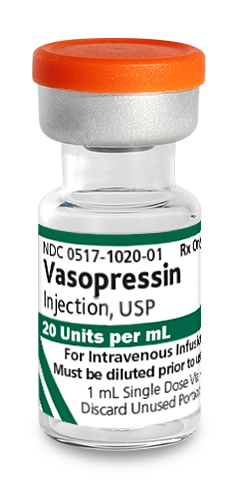
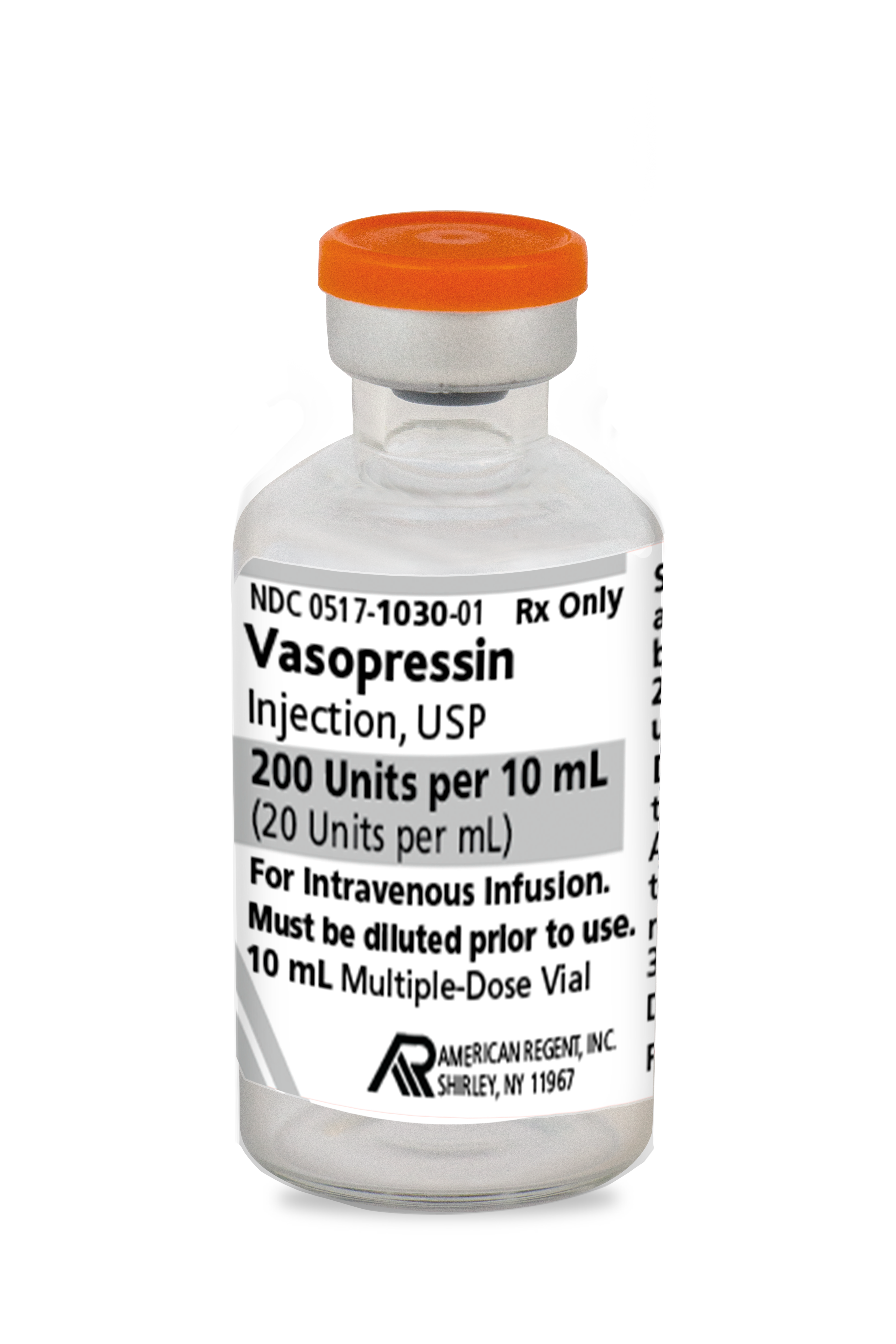
INDICATIONS AND USAGE
Vasopressin injection is indicated to increase blood pressure in adults with vasodilatory shock who remain hypotensive despite fluids and catecholamines.
For additional safety information, please see Full Prescribing Information.
You are encouraged to report adverse drug events to American Regent, Inc. at 1-800-734-9236 or to the FDA by visiting www.fda.gov/medwatch or calling 1-800-FDA-1088.
REF-1529 10/2024