Important Supply Update
American Regent is pleased to announce that Venofer is in stock, and we are able to fully meet demand.
Improving the lives of patients
Improving the lives of people and animals is what we do everyday. It is that simple mission; a commitment that drives us to develop and reliably deliver the essential medicines that patients need.
News Center
American Regent® Launches Gvoke VialDx™ (glucagon injection)
Shirley, NY – August 27, 2025: American Regent, Inc.® is pleased to announce the commercial launch and availability of Gvoke VialDx™ (glucagon injection).
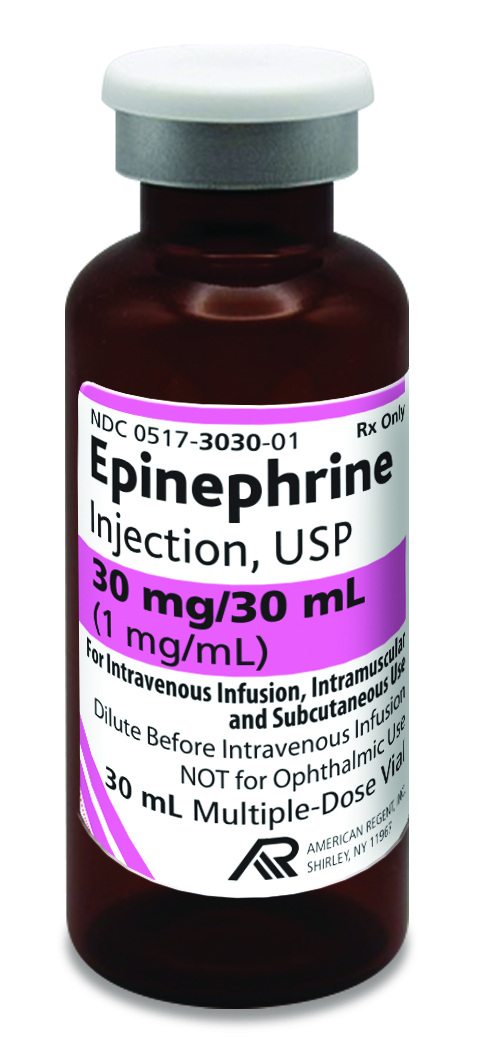
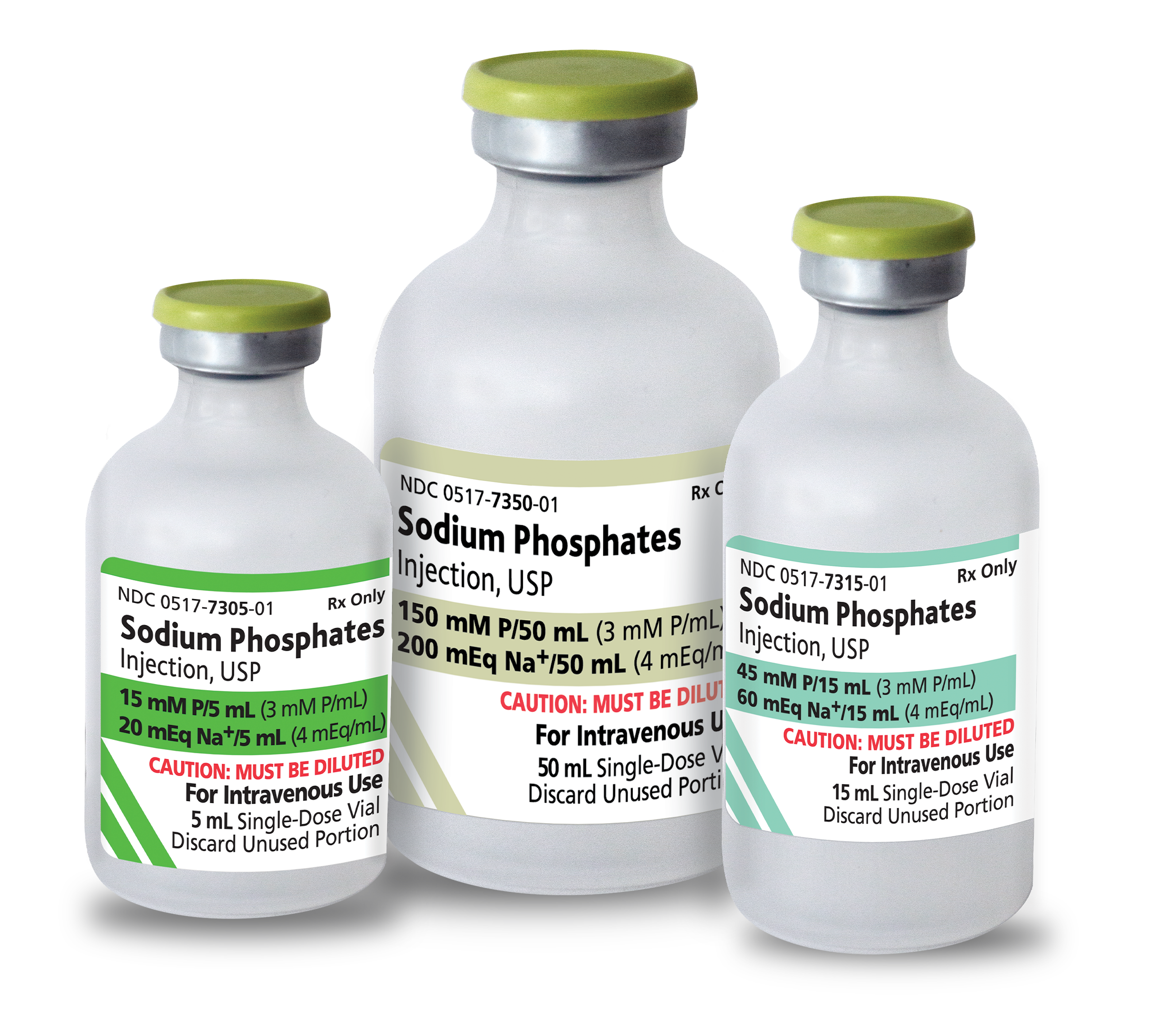
American Regent introduces Epinephrine Injection, USP in
30 mL; FDA-Approved and “AP” Rated
30 mL; FDA-Approved and “AP” Rated
Shirley, NY – October 15, 2024:
American Regent announces the launch and availability of 30 mL Epinephrine Injection, USP.
St. John’s University and American Regent Post-Doctoral Pharmaceutical Industry Fellowship Now Recruiting
Melville, NY – September 12, 2025:
Learn more about the St. John’s University and American Regent Post-Doctoral Pharmaceutical Industry Fellowship by downloading our brochure. Information on how to apply to our program as well as key deadlines are included.
Contact Us
By clicking Submit, you confirm that you accept our Privacy Policy and that you agree to your personal contact information being used to contact you and added to our database. If at any time you wish your personal information to be removed from the American Regent® database, please submit a message request to corpcommunications@americanregent.com.